What factors determine how quickly a battery can be charged? Researchers at the Karlsruhe Institute of Technology (KIT) are investigating this and other questions using computer-aided simulations. Microstructure models contribute to the discovery and investigation of new electrode materials. For sodium-nickel-manganese oxide as a cathode material in sodium-ion batteries, the simulations show changes in the crystal structure during the charging process. They lead to elastic deformation, causing the capacity to shrink. The researchers report in the journal npj Comutational Materials.
Research into new battery materials is not only aimed at optimizing performance and service life and reducing costs. It is also about reducing rare elements such as lithium and cobalt as well as toxic components. Sodium-ion batteries, which are based on similar principles to lithium-ion batteries but can be produced from raw materials that are sufficiently available in Europe, are considered promising. They are suitable for stationary and mobile applications. “Layered oxides such as sodium-nickel-manganese oxides are promising materials for the cathode,” reports Dr. Simon Daubner, group leader at the Institute of Applied Materials – Microstructure Modelling and Simulation (IAM-MMS) at KIT and corresponding author of the study. He is researching sodium-ion technology in the Cluster of Excellence POLiS (which stands for: Post Lithium Storage).
Mechanical stresses occur during fast charging
However, there is a problem with these cathode materials: sodium-nickel-manganese oxides change their crystal structure depending on how much sodium is currently stored. If the material is loaded slowly, everything is orderly. “Layer by layer, the sodium is removed from the material – like a parking garage that empties floor by floor,” explains Daubner. “But if it has to be done quickly, the sodium is pulled out from all sides.” This results in mechanical stresses that can permanently damage the material.
Researchers at the Institute of Nanotechnology (INT) and the IAM-MMS at KIT, together with scientists at the University of Ulm and the Center for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW), have now uncovered these relationships with the help of simulations and report on them in npj Computational Materials, a journal from the Nature portfolio.
Experiments confirm simulation results
“Computer models can describe different length scales, from the arrangement of atoms in electrode materials and their microstructure to the cell as the functional unit of each battery,” says Daubner. These combine microstructure models with slow charge and discharge experiments to investigate the layered oxide NaXNi1/3Mn2/3O2. The material exhibits several degradation mechanisms that lead to a loss of capacity. It is therefore not yet suitable for commercial applications. When the crystal structure changes, elastic deformation occurs. The crystal shrinks, which can lead to cracks and reduce the available capacity. As simulations carried out at INT and IAM-MMS have shown, this mechanical influence is so strong that it has a significant impact on how quickly the material can be loaded. Experimental studies at the ZSW confirmed the results.
The findings of the study can be partially transferred to other layered oxides. “Now that we understand the basic processes, we can devote further work to developing battery materials that are durable and can be charged as quickly as possible,” summarizes Daubner. This could make the widespread use of sodium-ion batteries a reality in five to ten years.
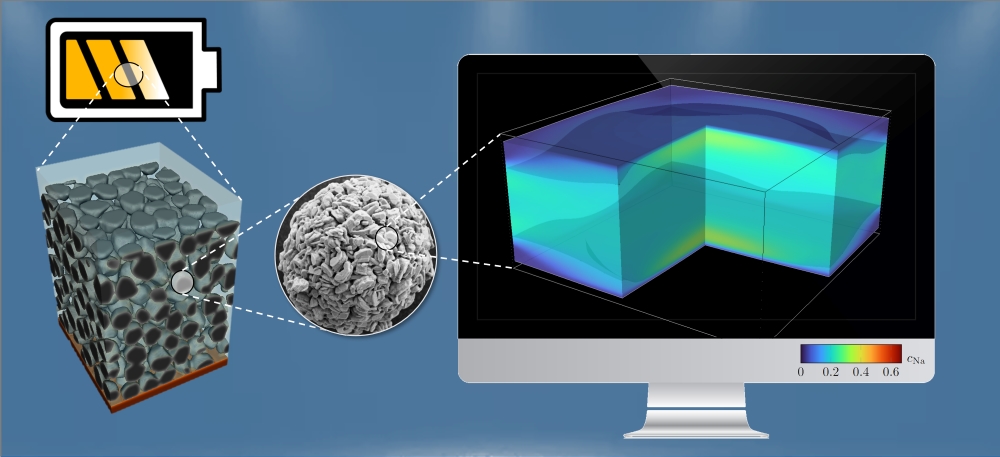
Top image: Section of a cathode layer (around 100 micrometers, left), consisting of spherical particles (diameter around ten micrometers, center), and simulation (right) of the sodium content in a sodium-nickel-manganese oxide crystal. Graphic: Simon Daubner, KIT